Brief introduction
Organic peroxides are organic compounds containing oxygen-oxygen bonds (---O---O---) (peroxy groups). It is characterized by decomposition and oxygen-containing free radicals after heating above a certain temperature, which is unstable and easy to decompose. It can be used as an initiator for free radical polymerization, an initiator for grafting reactions, a crosslinker for rubber and plastics, a curing agent for unsaturated polyester, and a molecular weight and molecular weight distribution regulator in the preparation of spinning-grade polypropylene.
When selecting organic peroxides, there are many factors to consider, such as:
half-life
Half-life time: The time required to decompose the remaining half at a given temperature. Most peroxide decompositions conform to the first-order reaction kinetics equation.
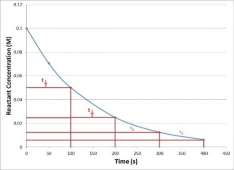
Half-life temperature: The temperature required to decompose half of the residue at a given time.
Half-life time and temperature are very important parameters for selecting the appropriate peroxide.
security
o Sensitive to heat and pollution
o Flammable
O May be sensitive to vibration and friction, such as benzoyl peroxide
o Decomposition releases heat and combustible gas and may spontaneously ignite
Maximum storage temperature
The maximum temperature at which the product can be stored, at which the product can be safely stored with as little activity reduction as possible.
Minimum storage temperature
o Some products have a minimum storage temperature.
Ø These products are sensitive to impact or friction and are generally diluted with solvents.
O When the temperature is lower than the minimum storage temperature, peroxides may precipitate, which is a risk.
Ø Safety, half-life temperature, half-life time, active oxygen, active ingredients, etc.
Shorter half-life times mean faster reactions and lower reaction temperatures.
Self-accelerating decomposition temperature
SADT
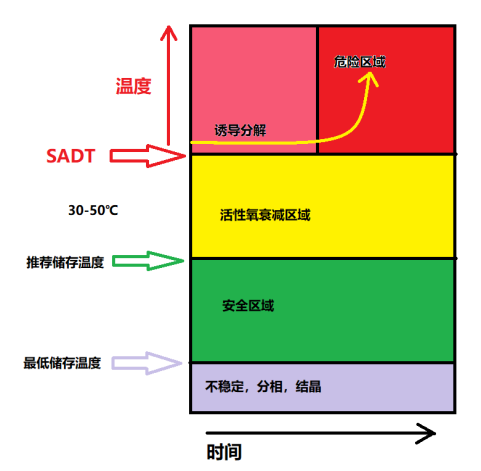
The lowest temperature at which the self-accelerating decomposition reaction of the test kit takes place.
The reaction produces flammable gases that may cause spontaneous combustion.
When the self-accelerating decomposition temperature is reached or exceeded, the time for the decomposition reaction to occur depends on how much temperature is exceeded and the time required for the reaction to release heat (heat released by increasing decomposition rates).
The content of active oxygen species
If A[O] < 1%, it is not defined as an organic peroxide
Incompatible substances
ØStrong acid, strong base and strong oxidant
Ø reducing agent, amines
Ø Transition metal salts, metal ions
Ø Some co-catalysts and accelerators
Compatible contact materials
Ø304 or 316 stainless steel (recommended)
o High density polyethylene, PTFE or glass inner layer
|
name Name |
Chemical name Chemical |
structure Structure |
molecular weight molecular weight g/mol |
Available oxygen content Active oxygen% |
content content,% |
Physical state |
Half-life, half life temp.°C |
Storage temperature, storage temp. °C | |||
|
|
|
|
|
|
|
|
10am |
1h |
1min |
least |
utmost |
|
ioxide BPO |
Benzoyl peroxide |
|
242.2 |
4.95 |
75 |
grain |
73 |
92 |
131 |
5 |
30 |
|
ioxide LPO |
Lauroyl peroxide |
|
398.6 |
3.95 |
98 |
Flakes |
64 |
81 |
117 |
On |
30 |
|
ioxide TBPB |
tert-Butyl benzoate peroxide |
|
194.2 |
8.07 |
98 |
liquid |
104 |
125 |
171 |
8 |
30 |
|
ioxide TBPO |
tert-Butyl peroxide 2-ethylhexanoate |
|
216.3 |
7.25 |
98 |
liquid |
77 |
94 |
128 |
On |
15 |
|
ioxide BECAME |
tert-Amyl peroxide 2-ethylhexanoate |
|
230.3 |
6.7 |
98 |
liquid |
75 |
92 |
126 |
On |
10 |
|
ioxide DTBP |
Di-tert-butyl peroxide |
|
146.2 |
10.72 |
98 |
liquid |
129 |
149 |
189 |
-30 |
30 |
|
Ioxide DTAP |
Di-tert-amyl peroxide |
|
174.4 |
8.81 |
96 |
liquid |
123 |
143 |
184 |
On |
30 |
|
ioxide CH |
Di-tert-butylcyclohexanone peroxide |
|
260.4 |
8.70 |
70 |
liquid |
97 |
116 |
155 |
On |
30 |
|
ioxide CH335 |
1,1-Di-tert-butylperoxide 3,3,5 trimethylcyclohexanone |
|
302.5 |
9.52 |
90 |
liquid |
96 |
115 |
153 |
On |
30 |
|
ioxide TAHP |
tert-amyl hydrogen peroxide |
|
104.2 |
13.05 |
85 |
liquid |
165 |
183 |
|
0 |
30 |
|
ioxide TBHP |
tert-Butyl hydrogen peroxide |
|
90.1 |
12.25 |
70/80 |
liquid |
172 |
200 |
260 |
0 |
30 |
|
ioxide TAEC |
2-ethylhexyl amyl carbonate peroxide |
|
260.3 |
5.84 |
95 |
liquid |
99 |
117 |
155 |
On |
30 |
|
ioxide TBEC |
2-ethylhexyl tert-butyl carbonate peroxide |
|
246.3 |
6.17 |
95 |
liquid |
100 |
121 |
166 |
On |
30 |
|
Ioxide KP |
4-Methyl-2-pentanone peroxide |
|
|
8.0 |
45 |
liquid |
/ |
/ |
/ |
On |
35 |
|
ioxide K11 |
Ketone peroxide |
Quartz stone is dedicated |
|
8.0 |
80 |
liquid |
/ |
/ |
/ |
On |
35 |
|
ioxide K22 |
Ester peroxide |
Quartz stone is dedicated |
|
8.0 |
80 |
liquid |
/ |
/ |
/ |
On |
35 |
|
ioxide K30 |
Ester peroxide |
Quartz stone is dedicated |
|
6.4 |
80 |
liquid |
/ |
/ |
/ |
On |
35 |
|
ioxide TBMA |
tert-Butyl cis butenedioate peroxide |
|
188.2 |
3.35 |
40 |
Paste-like |
82 |
104 |
150 |
0 |
30 |
|
ioxide 355 |
tert-Butyl peroxy-3,5,5-trimethylhexanoate |
|
230.3 |
6.95 |
60 |
liquid |
94 |
114 |
142 |
-20 |
25 |
|
ioxide TAPB |
tert-Amyl peroxide |
146.2 |
60 |
liquid |
100 |
120 |
162 |
-10 |
30 |

1) Organic peroxides are oxidizing and flammable substances, please dispose of them with caution, must be stored in a cool and ventilated place, and keep the container closed. Keep away from fire. Avoid contact with reducing agents, heavy metals, alkali metals, desiccants, strong acids, strong bases and other chemicals. Products cannot be weighed in the warehouse. The product should be stored at the temperature on the product label.
2) Explosive hazard, it is strictly forbidden to mix with the accelerator, must be added to the resin separately.
3) The peroxide must be stored in the original barrel, and the leftover peroxide cannot be put back into the packaging barrel.
4) When using peroxide, safety protective gloves and goggles must be worn. Timely diluted peroxide may still cause damage to the skin and eyes.
It is absolutely forbidden to smoke or work in the workshop or warehouse
Emergency response in case of accident
Eye:
When the eye touches peroxide, rinse with plenty of water for at least1-5 minutes and seek medical attention
Mouth:
When ingesting peroxide, induce vomiting and seek medical attention
Skin:
Rinse your skin with clean water or shower, remove clothes and shoes contaminated with peroxide, and seek medical attention
Divulge:
Small leaks: Mix with an inert substance (such as vermiculite or clean sand) and put in a safe place. Extensive leakage: Avoid contact with any ignition sources and flammable substances. Spray water to reduce steam, keep substances moist, and do not use metal tools and equipment. Prevent material from entering sewers, basements and control areas.
Fire:
Low fire: spray dry powder or carbon dioxide to extinguish, sprinkle water to prevent re-ignition. Fire: Keep a safe distance of 1 0-15 meters to fight the fire. *The above information is for reference only and should not be used to contravene laws and security regulations

1) Organic peroxides are oxidizing and flammable chemicals which must be handled with care. Keep container in a cool well ventilated place. Keep packaging closed when in use. Avoid exposure to heat, sparks, flame or any other ignition source. Avoid contact with dust. Keep away from chemicals such as reducing agents, metal compounds and accelerators. Keep the products in proper temp.
2) Risk of explosion. Never mix peroxides with accelerators directly.
3) Peroxides should be stored in original containers. Don’t return peroxides to original container but collect left peroxides carefully.
4) Wear safe glasses and gloves while handling peroxides.
Smoking and ignition are strictly prohibited in workshop
Emergency
Eyes:
Wash with plenty of water for at least 15mins. Ask for medical advice!
Ingestion:
Wash mouth treat with water. Seek medical advice.
Skin:
Wash with plenty of water or shower. Remove contaminated clothing and shoes.
Spill:
Little spill:mix with inert materials such as vermiculite or sands。 Large spill:avoid contact with any ignition sources and flammable materials. Use water spay to reduce vapors and keep them damp. Don’t use metal tools or equipment.
Fire:
Small fire:extinguish small fire with powder or carbon dioxide. Large fire: extinguish with large amounts of water. Applied from a safe distance 15m.
*the above data is only for reference. Our proposals can’t be used to contravene the law and safe rules.







